Some Definitions
Here are some definitions that will be helpful in understanding the background of this project and proir work.
Ternary Eutectic System - a system of three components, in which one phase is changed into three other phases at a certain temperature and ratio of the three components. For this project, a phase of liquid solution of Al, Ag, and Cu will be transformed into three phases Al, Al2Cu, and Ag2Al.
In this alloy, the eutectic ratios by mole fraction are 0.18 Ag, 0.69 Al, and 0.13 Cu at 773.6 K.
Ternary Phase Diagram - a 2- or 3-dimensional phase diagram that represents concentrations and phases of three components (a,b,c). There are four external variables that constitute a complete representation of a ternary system, ratio of any two components, temperature, and pressure. However, the ternary phase diagrams usually are depicted while one or two of the variable are kept constant. (How to read ternary phase diagrams, here and here)
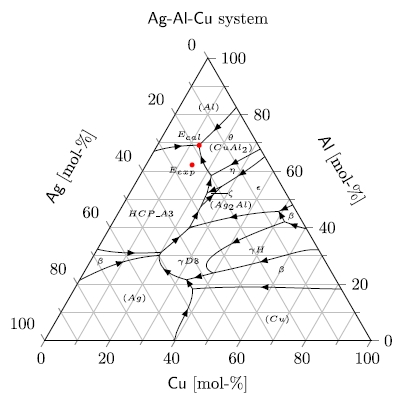 A 2-D liquidus (liquid state) diagram for Ag-Al-Cu system.
A 2-D liquidus (liquid state) diagram for Ag-Al-Cu system.
Directional solidification - a process of solidification within castings where the liquid metal solidifies in one direction and the interface of the solidifying metal is always in contact with liquid metal (feed metal).
Features in Ag-Al-Cu Microstructure
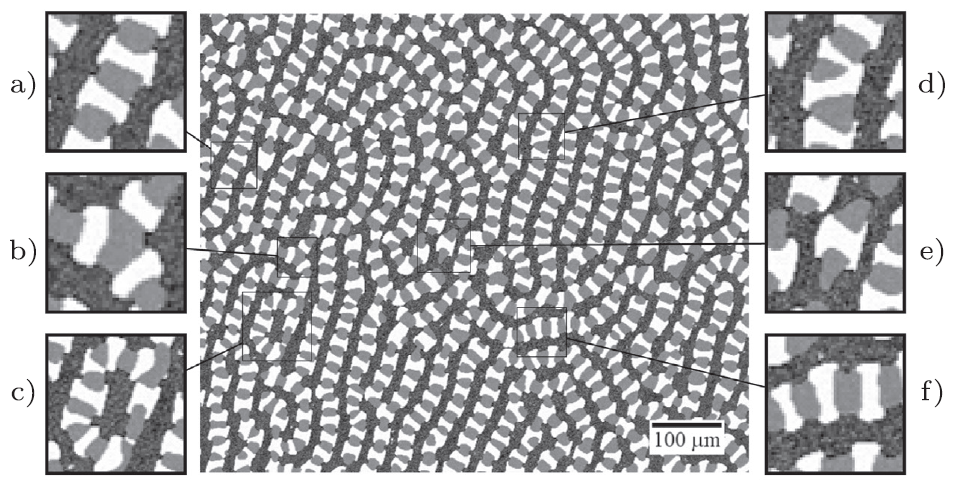
When the solidified Ag-Al-Cu alloy is formed, microstructure patterns form, consisting of three phases, as shown in the experimental micrograph:
- Al - black
- Al2Cu - grey
- Ag2Al - white
As the temperature of the liquid solution decreases, solid phases begin to form in the alloy. These phases do not necessarily form at the same time. The phase with the higher concentration will crystalize first and then the phase with next highest concentration will be next to solidify.
As the solution is solidified, the three phases form patterns, most notable a brick-like chains of Ag2Al (white) and Al2Cu (grey). The patterns can be categorized in six distinct features:
- a) Concave interfaces from Ag2Al to Al2Cu
- b) Triple interface between Ag2Al and Al2Cu
- c) Loop of Ag2Al and Al2Cu chain
- d) V-shaped formation of Ag2Al phase within Ag2Al and Al2Cu chain
- e) Al2Cu phase at end of Ag2Al and Al2Cu chain
- f) Sign change of curvature variation between Ag2Al and Al2Cu phases
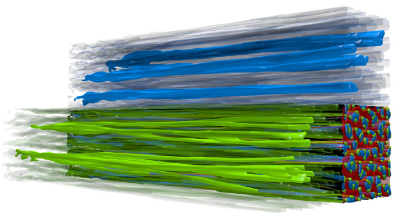
Finally, to visualize directional solidification, the following picture is a growth of the phases (left-to-right). In this case, the phases are colored the following way:
- Al - red
- Al2Cu - blue
- Ag2Al - green